Medical Device Assembly
Document What You Do, and Document You Did It
Medical device manufacturing presents some of the most challenging difficulties of all the industry segments. Unlike other sectors where compliance is conducted via private and customer audits, the FDA sets the standards and then provides the enforcement.
Off-the-shelf software is often used, but the manufacturer is responsible for the challenging task of ensuring the software will perform as intended, particularly when only some features of a package are used. Sequence helps its customers in their validation process of the software.

Benefits of Electronic Work Instructions for Medical Device Assembly
Objective Evidence
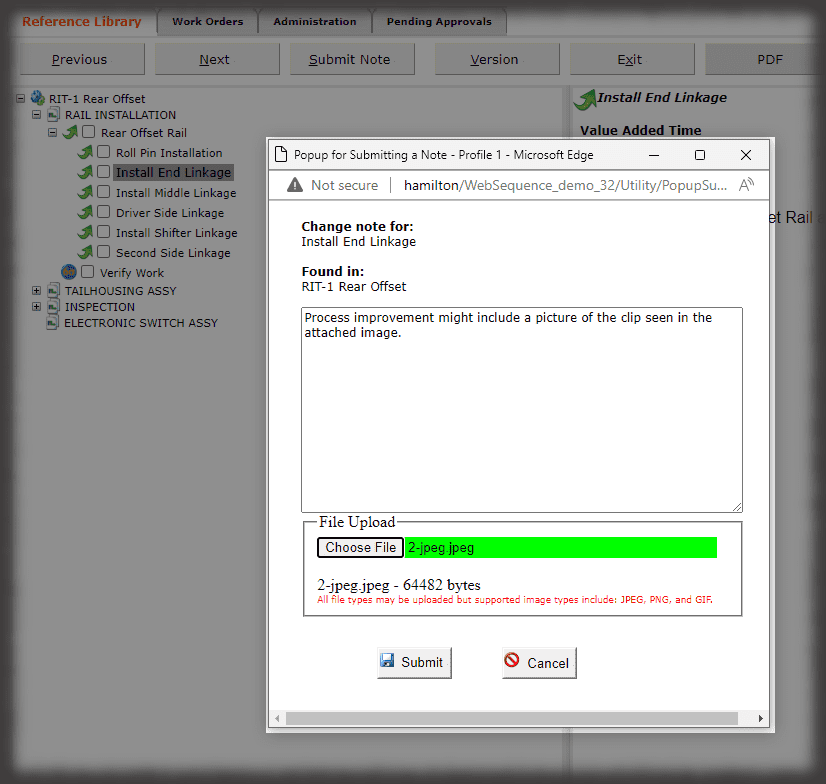
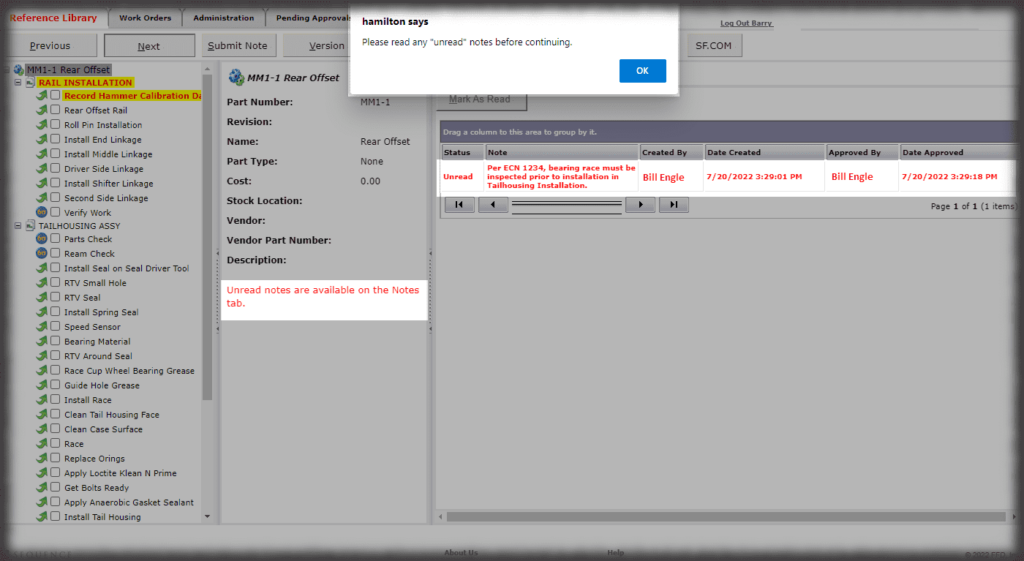
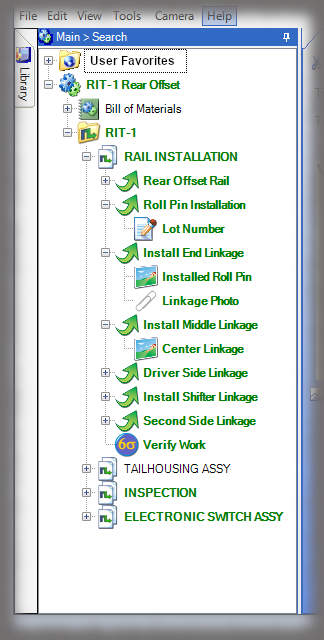
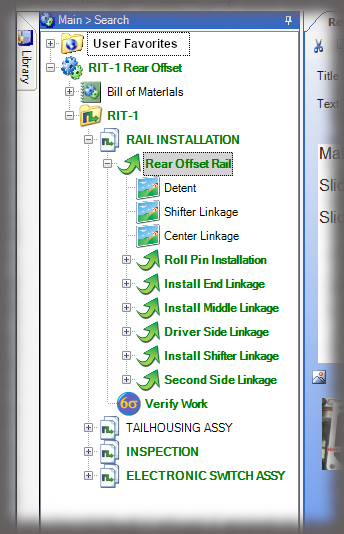
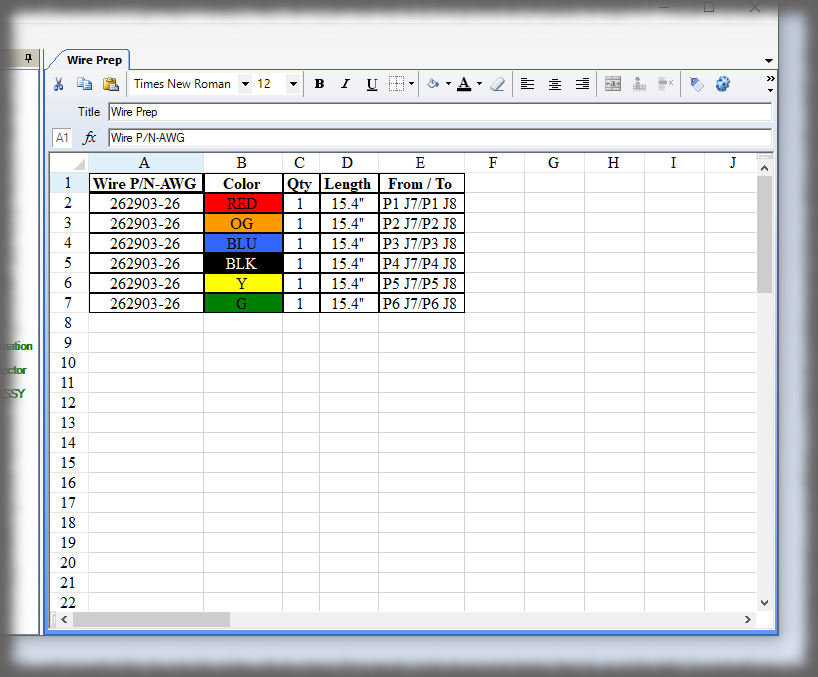
The hallmark of a device history record is the verifications, or signatures, of authenticated team members performing the work. While Sequence is not an MES solution, it does have “lite” capabilities in capturing other data that can be input, such as lot numbers or other traceable data, via keyboard, scanner or other keyboard emulating devices.
Device History Record
It has been said that half the cost of making a medical device is the cost of making it, and the other half is reporting that the device was manufactured the way the manufacturer said it was made.
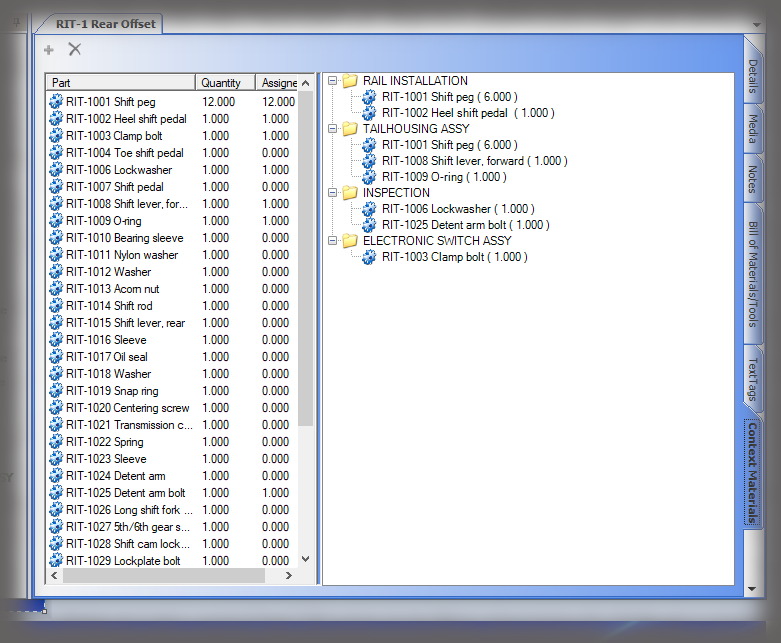
All data is stored in a query-able database making it easy to generate reports containing the actual instructions that were presented to the user as well as all data collected in manufacturing the device. Sequence provides many included PDF formats; but custom formats can be generated if needed, and there is the ability to create reports using third party reporting packages.
Version History
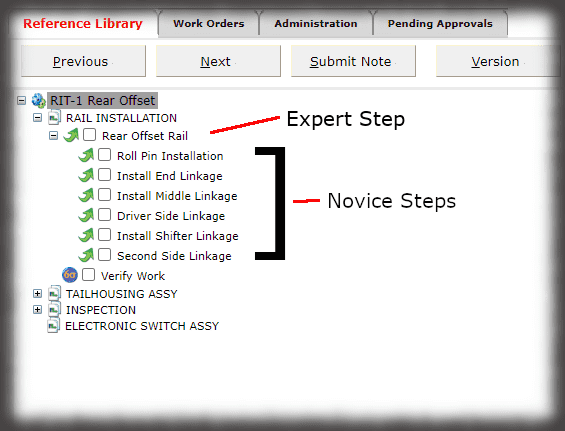
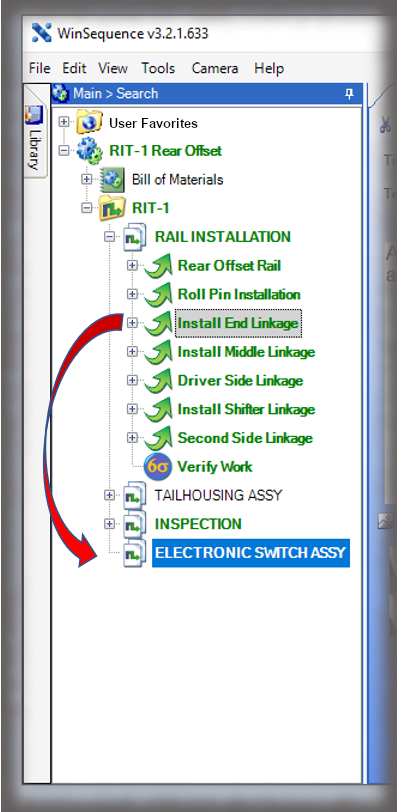
The core of the Sequence system is a database that maintains all versions of the process instructions. You decide how those instructions are approved via an approval routing with one or more individuals, and once approved those instructions are cast in stone until a new version is approved. Sequence provides historical preservation of all instructions in one package.
Electronic Records
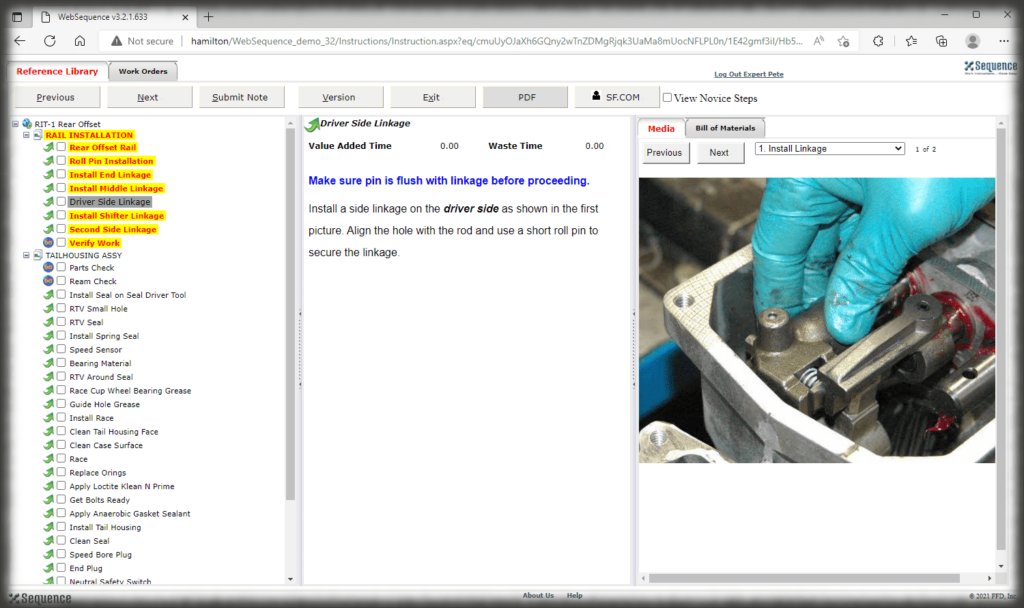
The security context of Sequence allows you to control who can log into the authoring and viewing interfaces and what those users can do—can they edit, approve, perform admin duties, or just view, for instance. The electronic work instruction, thus, eliminates paper, controls access, and ensures that users are presented with the correct revision of the instruction for the part they are making.
In addition to the role-based security, electronic records can be tuned to your specific requirements for compliance with 21CFR Part 11 rules. For example, you control which actions in Sequence require authentication and a number of settings with respect to passwords.
Industry Articles & Customer Success Briefs
Bruno Independent Living Aids Improves Work Processes
When you’re focused on making life easier for everyone else, sometimes you need help making your own life easier.

Single-User Desktop Application
Developed for the manufacturing company needing to rapidly author visual work instructions without the hassle.

Authoring Application to Create & Approve Content
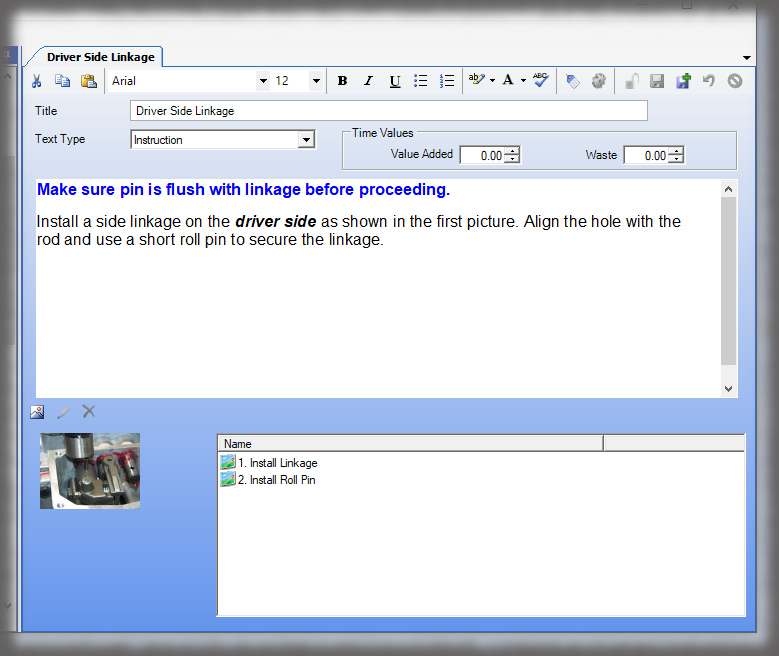
Our core product. Authors can quickly assemble rich, visual work instructions in a streamlined interface, then export to PDF.

Digital Deployment of Up-to-Date Visual Work Instructions
A dynamic electronic work instruction interface between engineering and the paperless manufacturing floor.

Digital Work Instructions Designed for Highly Repetitive Processes
An on-demand, scrollable, read-only shop floor access portal for paperless work instruction deployment.

Author within Sequence, Present within Your MES
A solution for organizations utilizing Manufacturing Execution Software (MES) that allows integration with the work flow.

Use BoMs, Ops, & Routings to Create Your Work Instructions Framework
A solution to ensure your electronic work instructions stay synchronized with your front-office software.