Work Instructions in Medical Device Manufacturing
Managing Work Instructions in an FDA World
Document What You Do, and Document You Did It
Medical device manufacturing presents some of the most challenging difficulties of all the industry segments. Unlike other sectors where compliance is conducted via private and customer audits, the FDA sets the standards and then provides the enforcement.
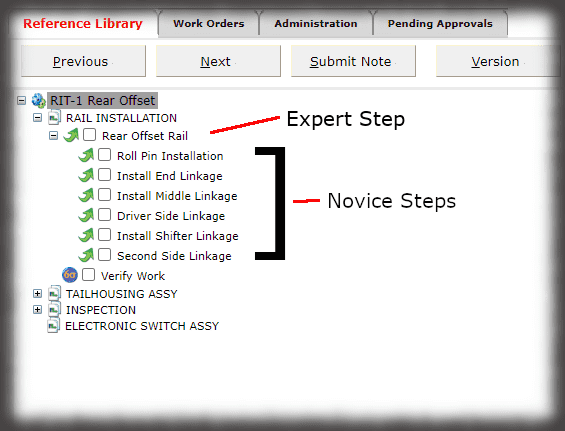
Off-the-shelf software is generally used, but the manufacturer is responsible for ensuring the software will perform as intended, particularly when only some features of a package are used. Sequence is digital work instruction software for manufacturing in medical devices.

Benefits of Electronic Work Instructions for Medical Device Assembly
Digital Work Instructions Save Paper and Printing Costs
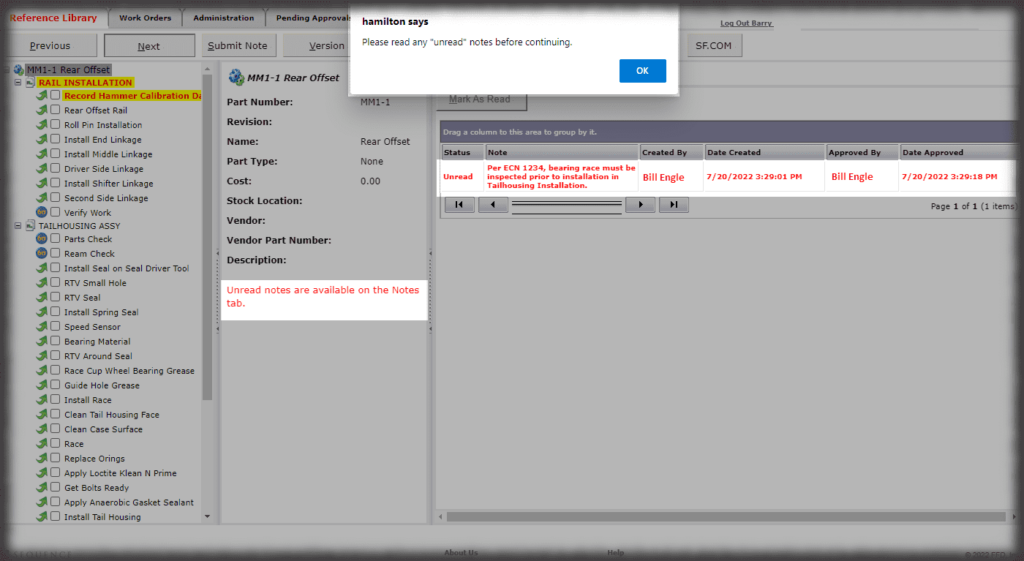
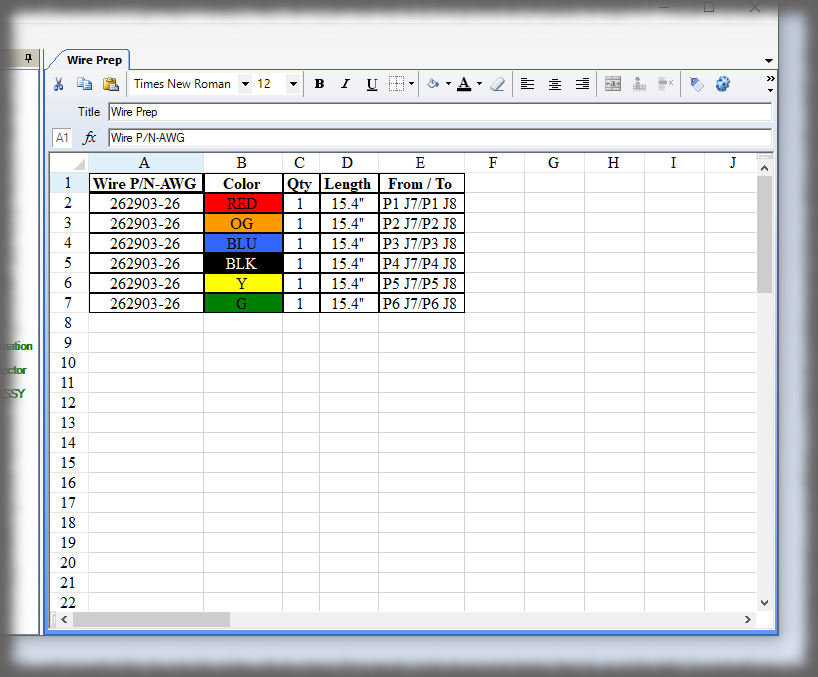
What is the cost of losing a paper traveler with signatures and data in manufacturing medical devices?
Medical device manufacturers want electronic work instructions in manufacturing for many reasons. First, you cannot afford to lose data. Second, it is “greener” and more secure to present instructions on a monitor as opposed to printing instructions and storing them in bulky binders. Finally, the data in a database can be “mined” later, unlike hard copy travelers that are scanned and put into the “iron mountain”.
Device History Record
It has been said that half the cost of making a medical device is the cost of making it, and the other half is reporting that the device was manufactured the way the manufacturer said it was made.
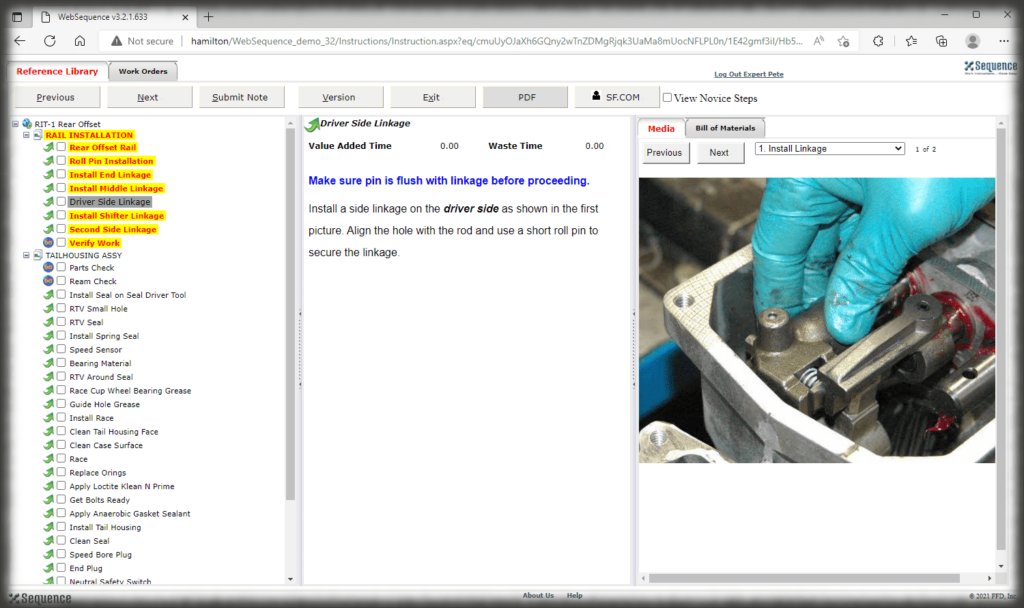
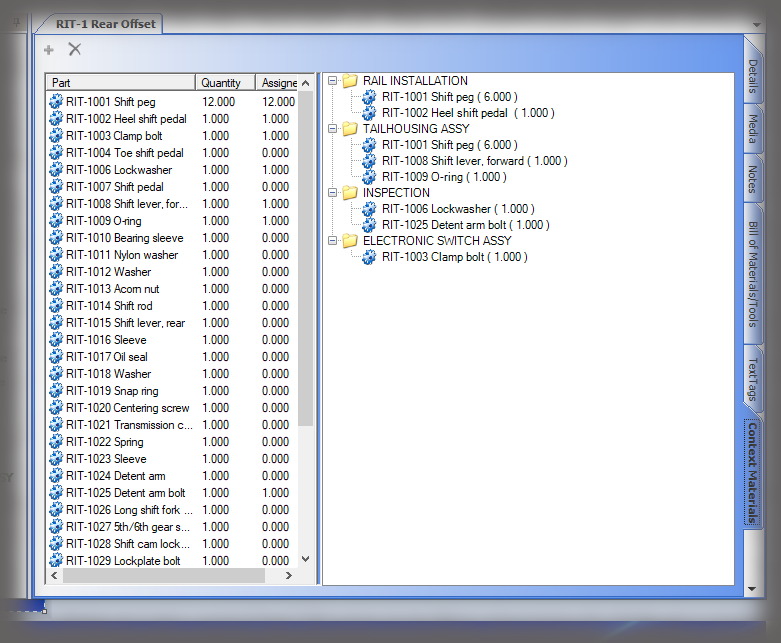
All data is stored in a query-able database making it easy to generate reports containing the actual instructions that were presented to the user as well as all data collected in manufacturing the device. Sequence provides many included PDF formats for reporting; but custom formats can be generated if needed, and there is the ability to create reports using third party reporting packages.
Version History
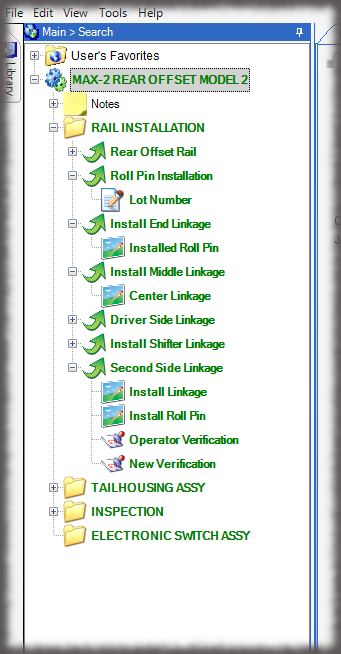
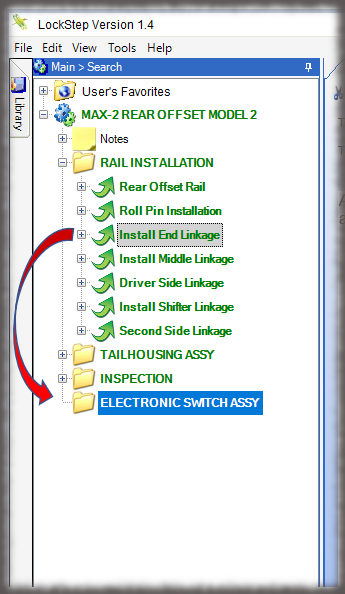
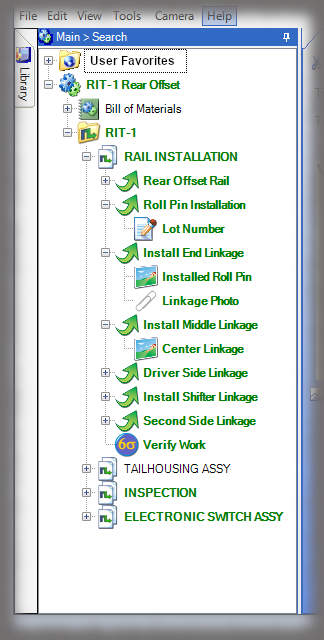
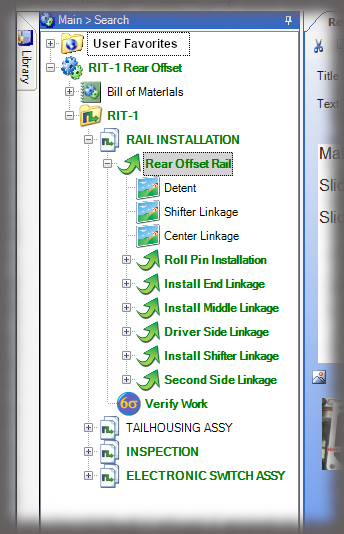
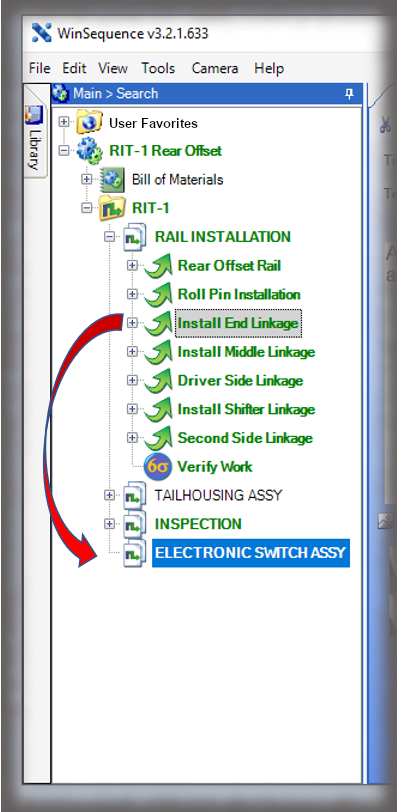
The core of the Sequence system is a database that maintains all versions of the process instructions. You decide how those instructions are approved via an approval routing with one or more individuals, and once approved, those instructions are cast in stone until a new version is approved. Sequence provides historical preservation of all instructions in one easy solution.
Electronic Records
The security context of Sequence allows you to control who can log into the authoring and viewing interfaces and what those users can do—can they edit, approve, perform admin duties, or just view, for instance. The electronic work instruction, thus, eliminates paper, controls access, and ensures that users are presented with the correct revision of the instruction for the part they are making.
In addition to role-based security, electronic records can be tuned to your specific requirements for compliance with 21CFR Part 11 rules. For example, you control which actions in Sequence require authentication, and you also control other aspects of password control (strength, expiration, failed retry attempts, etc.).
Change Notices & Compliance
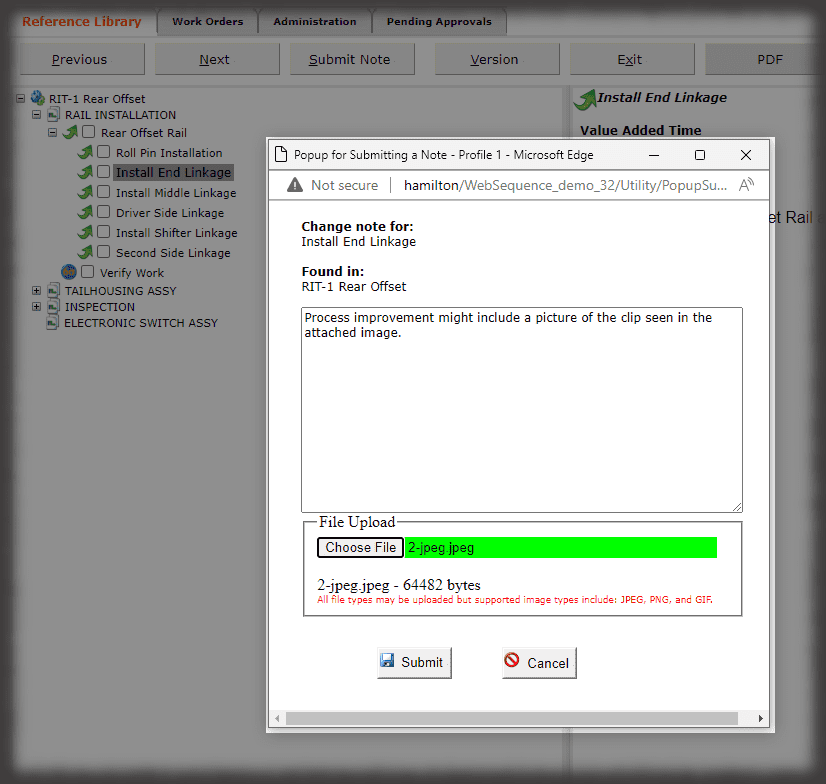
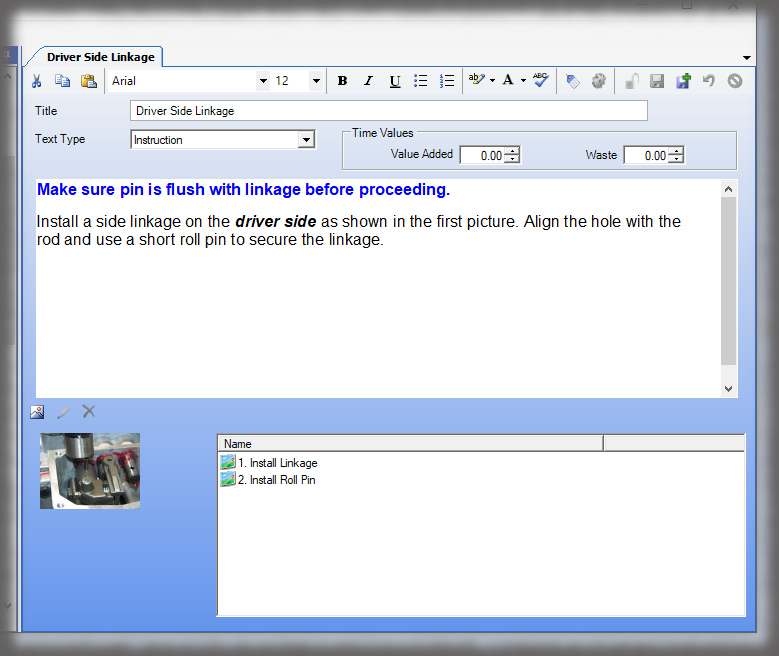
Whether it is a redline or a noncompliance it matters how your work instruction system works when things don’t go right in production. Beyond the standard benefits of version control, simple authoring and electronic instructions that span many industries, Sequence helps FDA regulated companies maintain the strictest traceability of rework and connection to your NC system. In the end, a work order contains the original instructions as well as any corrective actions directed by your review board. Rework instructions are authored in Sequence using the same tools that authors use to create work instructions, but they are reviewed, approved, and presented to users just the way the standard instructions are presented.
Resources
Reducing the Burden of Work Instructions on Manufacturing Engineers
In today's rapidly evolving manufacturing landscape, efficiency and productivity are critical. Manufacturing engineers play a vital role in ensuring smooth operation in production, but their effectiveness can be hampered by an excessive burden of writing and...
Avoiding Obsolete Work Instructions During ISO Audits
Maintaining up-to-date work instructions in manufacturing isn't easy, but it is critical for ensuring efficiency, consistency, and compliance with industry standards. Outdated work instructions often lead to errors, delays, and even safety hazards, which is why...
Quality Assurance & Work Instructions
An outline of how work instructions fit into an overall quality assurance program and digital work instructions support continuous improvement.

Single-User Desktop Application
Developed for the manufacturing company needing to rapidly author visual work instructions without the hassle.

Authoring Application to Create & Approve Content
Our core product. Authors can quickly assemble rich, visual work instructions in a streamlined interface, then export to PDF.

Digital Deployment of Up-to-Date Visual Work Instructions
A dynamic electronic work instruction interface between engineering and the paperless manufacturing floor.

Digital Work Instructions Designed for Highly Repetitive Processes
An on-demand, scrollable, read-only shop floor access portal for paperless work instruction deployment.

Author within Sequence, Present within Your MES
A solution for organizations utilizing Manufacturing Execution Software (MES) that allows integration with the work flow.

Use BoMs, Ops, & Routings to Create Your Work Instructions Framework
A solution to ensure your electronic work instructions stay synchronized with your front-office software.